Description
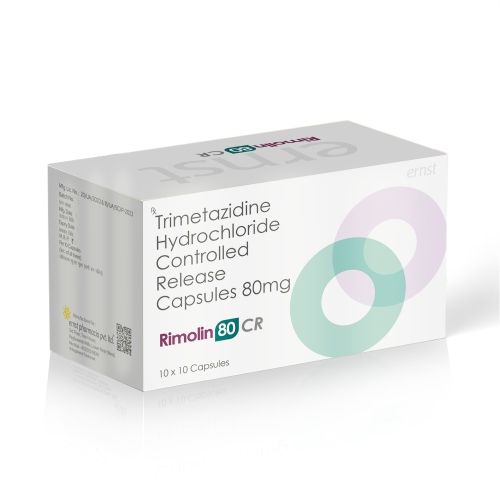
RIMOLIN 80 CR Capsules
Composition:- Trimetazidine Hydrochloride Controlled Release 80 mg
RIMOLIN 80 CR Capsules – Trimetazidine Hydrochloride Controlled Release Capsules 80 mg is a controlled-release formulation of Trimetazidine Hydrochloride, an anti-anginal (anti-ischemic) agent indicated for the management of stable angina pectoris. It works by optimizing cardiac cellular metabolism; enabling heart cells to function effectively even under reduced oxygen conditions. The controlled-release design provides a gradual, consistent release of the drug, ensuring prolonged therapeutic effect and stable plasma levels.
Key Points of Trimetazidine Hydrochloride Controlled Release Capsules 80 Mg – RIMOLIN 80 CR Capsules
• Metabolic Support for the Heart: Trimetazidine improves energy efficiency of cardiac cells without altering heart rate or blood pressure.
• Fewer Angina Attacks: Helps maintain energy balance in ischemic heart tissue, reducing the frequency and intensity of angina episodes.
• Long-Lasting 24-Hour Effect: The modified-release formulation allows once-daily dosing with continuous symptom control.
Can be Prescribed by:
• Cardiology
• General Medicine
• Geriatrics
• Critical Care Medicine
• Healthcare practitioner
Disclaimer: To be used under medical supervision only. Not intended for general public promotion. This content is meant for registered healthcare professionals only.



Reviews
There are no reviews yet.