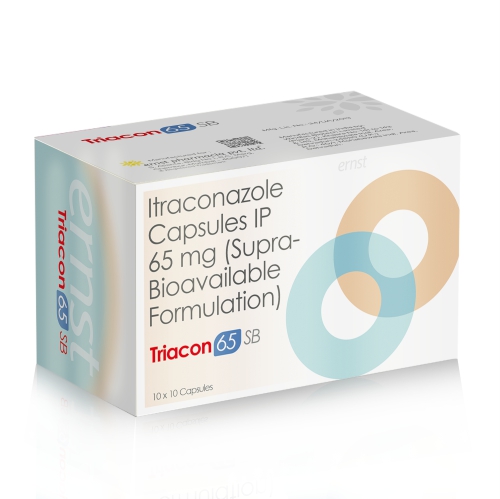
Description
TRIACON 65 SB Capsules – Targeted Antifungal Therapy with Enhanced Strength.
TRIACON 65 SB Capsules (Serba Technology) – Itraconazole 65 mg Capsules is a triazole antifungal agent that blocks the synthesis of ergosterol, an essential component of fungal cell membranes, resulting in disruption of fungal growth and cell death. It is widely used for systemic and superficial fungal infections, including dermatophytosis, onychomycosis, and vulvovaginal candidiasis caused by organisms such as Candida, Aspergillus, and Histoplasma.
Key Benefits of Itraconazole Capsules 65MG (Supra Bioavailable Formulation) – TRIACON 65 SB Capsules
✅ Broad-Spectrum Efficacy – Active against both superficial and systemic fungal infections.
✅ Effective in Resistant Strains – A reliable option where fluconazole or terbinafine resistance is noted.
✅ Convenient Oral Formulation – Capsule form ensures systemic absorption and ease of administration.
Recommended by specialists:
👨⚕️ Dermatologists – for resistant fungal infections of the skin, scalp, and nails.
👩⚕️ Gynecologists – for recurrent or chronic vulvovaginal candidiasis.
Indications
✅ Systemic fungal infections (aspergillosis, histoplasmosis, blastomycosis)
✅ Dermatophyte infections (tinea corporis, tinea pedis, tinea cruris)
✅ Onychomycosis (fungal nail infection)
✅ Oropharyngeal, esophageal, and vulvovaginal candidiasis
✅ Pityriasis versicolor





Reviews
There are no reviews yet.